预告|MDR-CE下代理商的机遇与挑战、迪玛克获证产品解析

课程获益
MDR认证是指欧洲医疗器械监管新法规(Medical Device Regulation,MDR)认证,旨在提高医疗器械的监管标准和保护患者的安全与健康。
MDR certification refers to the new European Medical Device Regulation (MDR) certification, which aims to improve the regulatory standards for medical devices and protect the safety and health of patients.
MDR在产品的风险管理、性能安全标准、上市前的临床评价以及产品上市后的警戒和监管等方面均提出了更高的要求。同时MDR对代理商资质、责任义务的要求也犹为严格。
MDR puts forward higher requirements in areas such as risk management, performance safety standards, pre-market clinical evaluations, as well as post-market vigilance and supervision. At the same time, MDR also sets strict requirements for the qualifications and responsibilities of distributors.
本次课程将重点分享MDR下制造商&代理商责任义务以及迪玛克获证产品解析
This course will focus on sharing the responsibilities and obligations of manufacturers and distributors under MDR, as well as an analysis of certified products by Demax.
直击问题
√相较MDD,MDR主要变化
√MDR制造商义务
√MDR代理商责任义务,注意事项
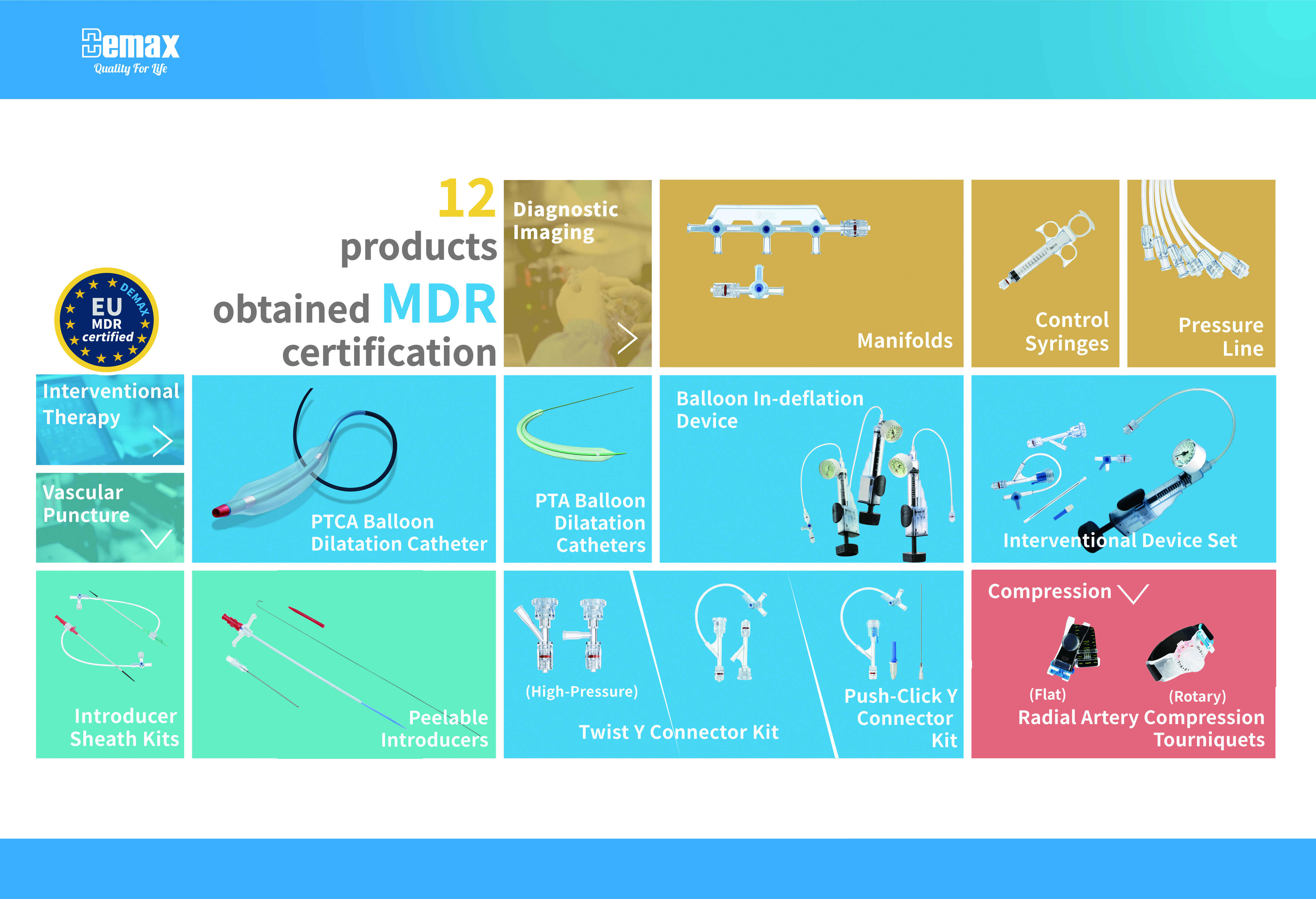
√迪玛克获证产品解析
√Compared to MDD, Major Changes in MDR
√MDR Manufacturer Obligations
√MDR Distributor Responsibilities and Cautions
√Analysis of Demax's Certified Products

迪玛克获证产品
迪玛克诚邀各位临床医生、合作伙伴共聚云端,探讨MDR-CE下代理商的机遇与挑战,拥抱新形势,畅聊新发展。
Demax sincerely invites all clinicians and partners to gather in the virtual space to explore the opportunities and challenges for distributors under MDR-CE, embrace new situations, and engage in discussions about new developments.
如需参与,欢迎联系迪玛克国际市场相关负责人,进入会议。
迪玛克产品知识云课堂,欢迎您的加入。
If you wish to participate, please contact the relevant person in charge of Demax International Market to join the meeting.
We're looking forward to seeing you.


 京公网安备 11011202002951号
京公网安备 11011202002951号